|

|
Dr. Melanie Pilkington
e-mail: pilkington@iac.unibe.ch
Tel.: ++41 31 631 42 45
Fax : ++41 31 631 39 93
go to:
Education
Research Experience and Interests
List of Publications
After work Interests and Activities
EDUCATION:
1991-1995 University of Kent at Canterbury
PhD in Synthetic Organic Chemistry. Thesis Title: An Exploration of the Uses of Cyclic Sulphamidates and Cyclic Sulphate Esters in Organic Synthesis, and an Investigation of Attractive Molecular Interactions by X-ray Crystallography.
My PhD research is divided into two main areas. The first describes attractive molecular interactions involving carbon-carbon triple bonds. The second area concerns the preparation and synthetic applications of cyclic sulfamidates and cyclic sulfate esters.
1989-1990 Erasmus Student at the Faculty of Industrial Chemistry, Bologna, Italy
1987-1991 University of Kent at Canterbury
Bachelor of Science, BSc. (Hons) in Biological Chemistry with Studies in Italy
|
RESEARCH EXPERIENCE AND INTERESTS
My research interests are based around the study and understanding of attractive, non-covalent molecular interactions (hydrogen bonding, electrostatic and van der Waals contacts) which have important and wide-ranging applications in both the living and non-living world. The study and understanding of such interactions requires skills and training of a multi-disciplinary nature. These interactions are present throughout many areas of organic, inorganic, physical and analytical chemistry, as well as being of fundamental importance to the design and functions of biological molecules. An improved understanding regarding the energies and stereochemical characteristics of these interactions could contribute for example to the design of artificial receptor molecules, which are capable of binding substrates in a stronger and more selective manner. During the last decade chemistry has expanded from the molecular level to the supramolecular level, and chemists are now using non-covalent, weak intermolecular interactions to spontaneously assemble large molecular structures or "supermolecules". Functional supermolecules have many potential applications for example, in the developement of nanoscale devices such as molecular switches for the electronics industry, as well as for the synthesis of artificial tailor-made enzymes which could function as efficiently as their biological counterparts.
1999 (May) Department of Chemistry and Biochemistry, University of Bern, Switzerland.
Postdoctoral research assistant in the group of Professor Silvio Decurtins. Current research focuses on the design and synthesis of new building blocks for the self assembly of supramolecular polymetallic systems with coupled magnetic and photophysical properties.
1997-1999 Chemistry Department, University of Cambridge, England
Postodoctoral research assistant in the group of Professor Tony Kirby. Research involved:
The design and synthesis of a more efficient enzyme model for Lysozyme
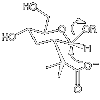
One of the major intellectual challenges of our time is a proper understanding of how enzymes work. At one level we can 'explain' enzyme catalysis, but our current level of understanding fails with the more severe practical test- that of designing and making artificial enzyme systems with catalytic efficiencies which rival those of their natural counterparts. The aim of this research is to work towards a more detailed understanding of the chemistry of enzyme catalysis, by testing recent ideas as to the way in which enzymes catalyse particularly slow reactions of fundamental importance. This research project involved the design and synthesis of model systems for glycosyl transfer with two catalytic groups, each operating with optimal efficiency. The objective of the project which is still ongoing is to develop systems in which general acid catalysis is efficient to make typical poor leaving groups good enough to make nucleophilic catalysis efficient. The synthesis of the glucopyranose model system shown below, where a suitable nucleophile (carboxylate anion) is placed strategically, so that it an displace good leaving groups (OR, where R=Ph) has been achieved (8 steps from Tri-O-acetyl-D-glucal). Progress has been made to introduce a general acid so that it converts a good leaving group into a much better one, and this chemistry is currently being extended to incorporate poorer leaving groups (OR, where R = alkyl).
1991-1997 Institute for Chemical and Mineralogical Crystallography, University of Bern, Switzerland
Postdoctoral research assistant in the group of Professor H.B. Bürgi. Research involved:
The Synthesis, Structure and Solution Spectra of Five coordinatre Platinum (II) Complexes of 1,4,7-trithiacyclononae incorporating diimine ligands.
Ligand exchange reactions of square planar d8 metal complexes usually proceed by nucleophilic attack at the metal centre leading to a five coordinate adduct with square pyramidal structure. This intermediate rearranges via a trigonal bipyramidal structure to a second pyramidal intermediate with the leaving group in the apical position. Dissociation of the leaving group finally produces the square planar product molecule with one ligand exchanged. The object of this research was to synthesise, and investigate a series of Pt(II) complexes of the type [Pt(N-N)[9]aneS3][PF6]2. The crystal structures of these molecules feature a five coordinate metal centre in a distorted square pyramidal geometry. The bidentate ligand and two of the sulfur atoms of the [9]aneS3 are bound in square planar fashion, whereas the third sulfur of the thia crown ether occupies the apical position (Figure 2, right). The molecule in the crystal can be considered as a substrate (Pt) reactant (S) pair in spatial proximity (fixed by [9]aneS3) that is about to start its journey toward a five coordinate transition state. Solution NMR studies at different temperatures indicate that these Pt(II) complexes undergo rapid intramolecular rearrangements which are similar to the nuclear motions associated with the formation and decay of the transition state in an associative substitution reaction of a square planar complex, Temperature dependent solution UV-VIS absorption spectra show unusually red shifted MLCT bands with decreasing temperature. These shifts have been assigned to a previously unobserved ground state equilibrium between exodentate and an endodentate conformation of the [9]aneS3 ligand in solution (Figure 2).
 |
| Exodentate |
Endodentate |
| Figure 2 |
Molecular Recognition in Fullerene Chemistry
The convex shaped surface of fullerenes can be recognised by concave shaped groups, such as corannulene or bent anthracene fragments via van der Waals interactions. The larger the surface of the recognition group, the better the interaction. The aim of this research was to co-crystallise C60 and C70 molecules with various concaved shaped molecules and to investigate self recognition motifs by single crystal X-ray crystallography.
|
LIST OF PUBLICATIONS
- M. Pilkington, H.B. Bürgi, H. Nikol, H.B. Gray, F.W.M. Vanhelmont and H. U. Güdel, Inorg. Chem, 1999, manuscript in preparation.
- M. Pilkington, H. B. Bürgi, H. Nikol and H.B. Gray, Inorg Chem., 1999, manuscript in preparation.
- T. Ozturk, M. Pilkington, C.R. Rice, D.A. Tranter, R. Hoezl, J.D. Wallis, M. Qayuum and P. Kathirgamanathon., J. Electrochem. Soc., 1999, submitted for publication.
- B. Therrien, T.R. Ward, M. Pilkington and C. Hoffman, Organometallics., 1998, 17, 330.
- J. Hauser, M. Pilkington, C. Hoffman, S. Capelli and H.B. Bürgi., Acta Cryst. 1997, C53, 1719.
- F. Leuquin, T. Ozturk, M. Pilkington and J.D. Wallis., J. Chem. Soc. Perkin. Trans 1. 1997, 3173.
- J. Howard, M. Pilkington, G. Smith and J.D. Wallis., J. Chem. Soc. Perkin. Trans 2, 1996, 1849.
- S. Larson, M.Pilkington and J.D. Wallis., J. Chem. Soc. Chem. Commun, 1995, 1499.
- M. Pilkington, S. Tayip and J.D. Wallis., J. Chem . Soc. Perkin. Trans 2, 1994, 2481.
- P. Gritsione, M. Pilkington, J.D. Wallis and D.C. Povey., Acta Cryst, 1994, C50, 763.
- M. Pilkington and J.D. Wallis., J. Chem. Soc. Chem. Commun, 1993, 1857.
|
|
|

